
Sara Skoog started college as pre-med, switched her major to English and has spent 20+ years writing about medical education and health care. Hobbies include baking, crosswords and not watching sports.
Author Page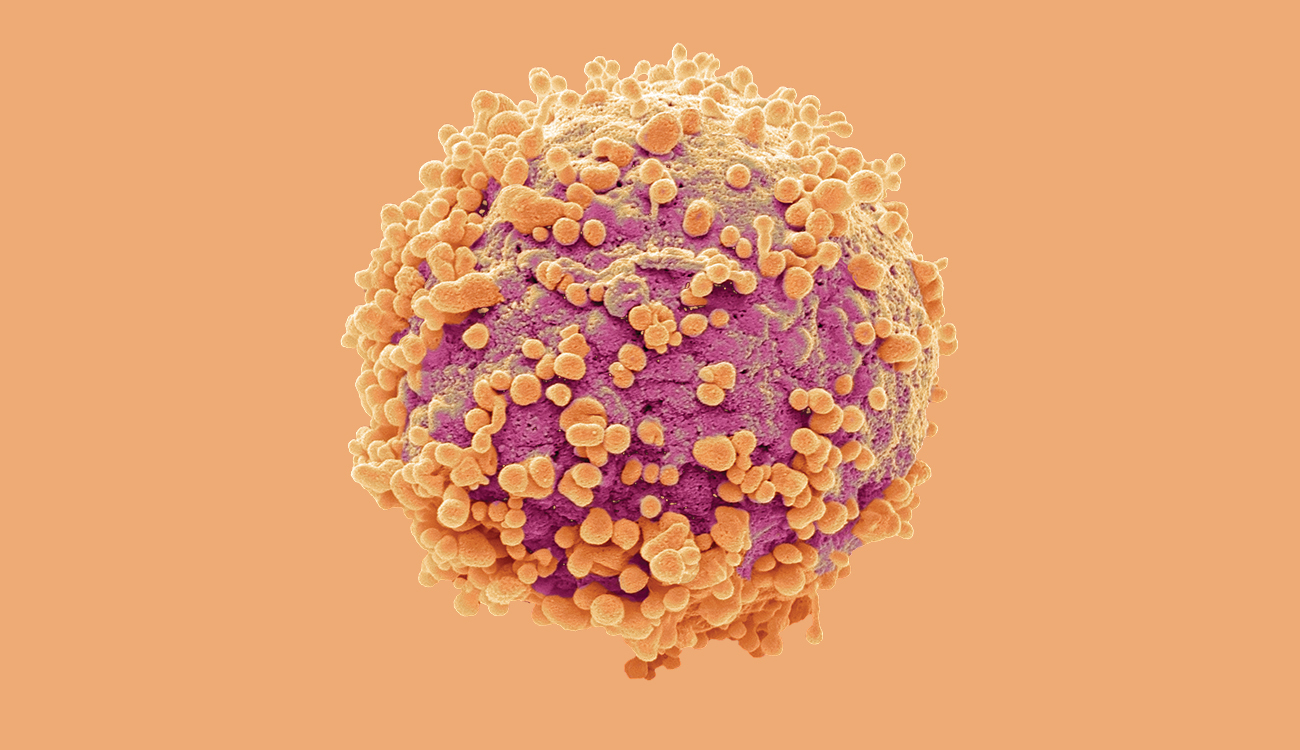
RFU’s bioscience incubator accelerates the development of new therapies
Image: Steve Gschmeissner / Science SourceBig ideas need a place and space to grow, to transform from a hypothesis into practical solutions for real-world problems. When the proposed solution is something novel, such as better therapeutics for aggressive forms of cancer that resist most treatments and are highly lethal, the path forward requires clearing a seemingly endless series of operational and regulatory hurdles.
For a researcher in a newly established bioscience company with minimal personnel and financial support, just getting your big idea off the ground takes lots of resources and time — resources you don’t have, and time that patients with lethal diseases may not have either.
Bioscience incubators, such as Rosalind Franklin University’s Helix 51, exist to connect start-ups and early-stage companies with the experts and resources they need to accelerate the development of new therapies now. Not after a lengthy search for affordable office space or months of haggling over leasing fees for expensive equipment. Now.
It can take 12 years or more for a new type of cancer drug to develop from the discovery phase to FDA approval and commercialization. That decade might as well be a million years for patients facing cancer diagnoses with statistically low survivability. While incubators can’t guarantee instant success or eliminate all the red tape, they can immerse researchers in an environment that puts resources in much closer reach.
BRAIN MATTERS
Apoptosis, the process of programmed cell death, happens when a cell becomes old or damaged. As in this brain cancer cell, the cell becomes spherical as its cytoskeleton, which holds cell shape, is digested, and orange blebs form on its surface.
How close? The molecular biologist next door might have access to a lab that can analyze your samples in days and walk the results down the hall, saving time and money.
Helix 51 is part of the bioscience corridor in Lake County, Illinois, counting among its neighbors 122 bioscience companies, including Abbott, AbbVie, Pfizer, Fresenius Kabi, Baxter and many others. With 33,000 life-science jobs in Lake County, this is fertile ground for ideas to take root and rise up.
Every person’s cancer journey is different, and every body has its own reaction to the disease and the methods available to treat it. Cancer is personal, and how we treat it is becoming personal, too.
Researchers are creating directed therapies that can be potent enough to kill cancer cells, but are also programmed to recognize and spare healthy cells. This personalized approach can improve the patient’s quality of life by reducing or even eliminating debilitating side effects that accompany more traditional treatments.
Clinical-stage biotech company Monopar Therapeutics (Nasdaq: MNPR) is drawing on the resources and expertise of the incubator to develop targeted treatments. Monopar has several radiopharmaceutical drugs under development in discovery, preclinical or early clinical phases (see sidebar), including several specific to the imaging and treatment of advanced solid cancers, such as breast and pancreatic cancers.
Creating such targeted therapies requires expertise from multiple fields, and Helix 51 provides ready access to academic specialists and advanced scientific research equipment.
“Being here gives us access to RFU’s scientific experts, resources and facilities, which is helping us accelerate development and optimization of our radiopharmaceuticals to treat aggressive forms of cancer,” said Andrew Cittadine, chief operating officer at Monopar.
Incubators foster biomedical advances through shared knowledge and resources, speeding the development of desperately needed therapeutics to treat devastating diseases. They nurture ideas that grow into new hope for those who need it most.
RFU’s Helix 51 bioscience incubator is the launchpad for these five early-stage companies pursuing treatments for patients with some of the most aggressive cancers.
Targeting Brain Cancer: Unlike traditional chemotherapy, which poisons both cancerous and healthy cells, oncolytic viruses specifically target and kill cancer cells. U.P. Oncolytics’ direct therapy minimizes collateral damage, resulting in fewer side effects. Injectable oncolytic viruses, unlike other treatments, can bypass the blood–brain barrier and reach the brain, avoiding unnecessary surgery.
Aiding Immunity: Few effective treatments exist to combat cancers such as glioblastomas and leukemias. ARTEC Biotech’s approach converts stem cells into natural killer (NK) immune cells and modifies these cells to fight cancers. This immunotherapy treatment is safer than other immunotherapy approaches.
Boosting Natural Defenses: Gencell, a Mexican biotech and Helix 51’s first international resident, is developing stem-cell therapy for diseases, including cancer. Gencell and ARTEC plan to collaborate to convert stem cells into natural killer cells to fight cancer as part of immunotherapy. This treatment would capitalize on the body’s natural defenses and precisely target cancerous cells while leaving normal cells intact.
Radiating Cancer Cells: Radiopharmaceuticals locate tumors and diagnose cancer by binding to specific molecules on the surface of cancerous cells. Monopar Therapeutics designed a radiopharmaceutical drug that targets cancer cells, killing them with specific amounts of radiation.
Impeding Fibrosis: Overgrowth of connective tissue, known as fibrosis, can lead to several autoimmune diseases and solid tumors. BLR Bio is developing a new class of peptides that impede the development of fibrosis. The company is initially targeting pancreatic cancer, a deadly disease with few effective treatments and a five-year survival rate of only 50%.
Development of a new drug involves a rigorous process divided into multiple stages before the drug can be considered for regulatory approval and authorization to market:
Discovery: New compounds or technologies are developed in the lab targeting a specific disease pathway.
Preclinical research: Laboratory testing measures the efficacy, safety and potential side effects of the drug.
Clinical research: Testing with human subjects via strictly controlled and regulated protocols.
Advance world-class research into innovations that improve patient lives and well-being.